New method allows for realistic, 3D model of this human synapse
The neuromuscular junction—where nerves and muscle fibers meet—is an essential synapse for muscle contraction and movement. Improper function of these junctions can lead to the development of progressive neuromuscular diseases, some of which have no effective treatment (like Lou Gehrig’s disease). Now, NIBIB-funded researchers have found a way to model the human neuromuscular junction by growing these synapses in a lab, which could accelerate novel treatments for neuromuscular diseases.
“Traditionally, studies of the neuromuscular junction rely on small animal models, but the human synapse has key differences, ultimately limiting the utility of animal studies,” said David Rampulla, Ph.D., director of the division of Discovery Science & Technology at NIBIB. “Here, the study authors have developed a method to evaluate the neuromuscular junction using human 3D tissue models, which enables a more accurate representation of human disease.”
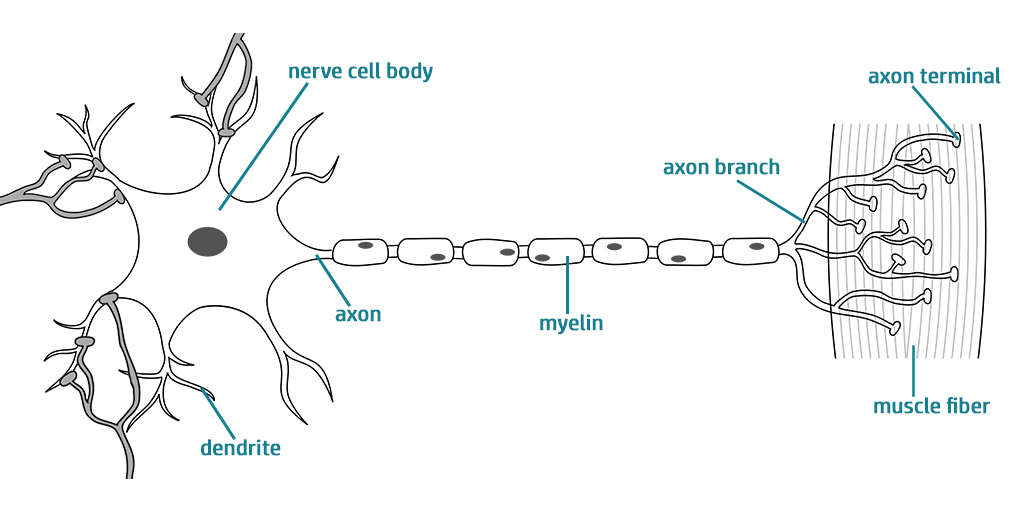
The neuromuscular junction is essentially comprised of two different types of cells: skeletal muscle cells and a type of nerve cell called motor neurons. Motor neurons are covered with ion channels, which open in response to electrical signals from the brain. Once these ion channels are open, a series of cascading reactions allows the signal to reach the skeletal muscle cells, which ultimately results in muscle contraction. Therefore, in order to generate a 3D model of the human neuromuscular junction, the researchers had to acquire and grow both these types of cells. They accomplished this by either using a muscle biopsy from a donor, from which muscle cells were isolated and neuron cells were genetically derived, or by using human stem cells which were genetically modified to make both cell types.
Once they had acquired the necessary cells and grew their models, the researchers needed to find a way to measure how the neuromuscular junction was functioning. To do this, they genetically modified the motor neurons to express ion channels that open when exposed to blue light. This approach, called optogenetics, allows the researchers to precisely stimulate the motor neurons with light and then analyze the resulting muscle contractions. In this way, the researchers can directly control the neuromuscular junction and quantify changes in its function.
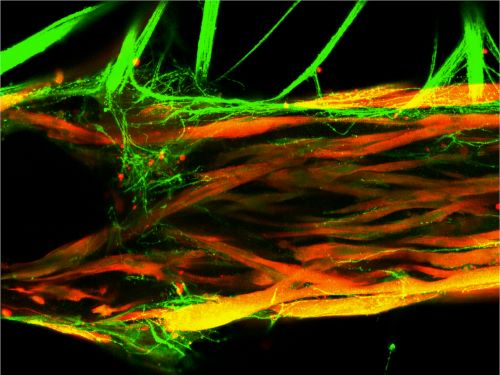
To fully control and monitor these photosensitive models, the researchers grew the motor neurons and muscle cells in separate but adjacent chambers, allowing axons (the thin filaments that carry nerve impulses) to “sprout” from the neurons and travel toward the muscle cells. The researchers then designed a custom optoelectronic platform to both stimulate—and film—their models. Then they could analyze how well the muscle tissues were contracting in response to stimulation (from the blue light).
Top: Muscle cells are grown in a chamber with two flexible pillars, while motor neurons are grown in a separate but connected chamber above (not shown). After approximately two weeks, the muscle tissue becomes innervated (contains axons). When the motor neurons are exposed to blue light pulses (flashes in upper left corner), the muscle tissue contracts. Bottom: The contractions of the muscle tissue are measured in response to the light pulses. If the light pulse triggers a contraction, it is indicated in green; if the light pulse does not trigger a contraction, it is indicated in red. Credit: Vunjak-Novakovic lab at Columbia University.
“Beyond studying the healthy neuromuscular junction, our method can also be adapted for patient-specific models, to either diagnose disease, or eventually to evaluate new treatment modalities for hard-to-treat neuromuscular conditions,” explained senior study author Gordana Vunjak-Novakovic, Ph.D., University Professor and Mikati Foundation Professor of Biomedical Engineering at Columbia University in New York.
Indeed, in their study, the researchers exposed their models to serum taken from patients with myasthenia gravis, an autoimmune disease that attacks the neuromuscular junction and causes muscle weakness. The researchers found that exposure to this serum drastically impaired the function of their human models, demonstrating that their system can be used to model human disease.
“Our platform demonstrates that we can quantify the function of both healthy and diseased models of the human neuromuscular junction in an unbiased, reproducible manner,” noted Vunjak-Novakovic.
“New approaches for the treatment of neurodegenerative diseases are sorely needed, as decades of research have resulted in limited therapeutic advances,” said first study author Olaia F. Vila, Ph.D., who is now a scientist at Amgen in San Francisco. “We hope that our models of the human neuromuscular junction can be used in the near future to aid drug discovery and new treatments for some of these diseases.”
This study was reported in Biomaterials.
This work was supported by two grants from NIBIB (EB025765 and EB027062) along with a grant from the National Institute of Environmental Health Sciences (NIEHS; grant U01ES032673) and an award from the U.S. Department of Defense (DOD; award number W81XWH-18-1-0095). One study author received support from the National Heart, Lung, and Blood Institute (NHLBI; grants R01HL130533, R01HL135358, and P01HL146366), the National Eye Institute (NEI; grant R01EY028249), and the National Institute on Aging (NIA; grant RF1AG072052).
Study reference: Olaia F. Vila et al. Bioengineered optogenetic model of human neuromuscular junction. Biomaterials, Volume 276 (2021). https://doi.org/10.1016/j.biomaterials.2021.121033
About the artwork (top graphic): This graphic was adapted for this highlight and is licensed under the Creative Commons Attribution-ShareAlike 4.0 International license.
