The technology may have broader applications
If you’ve been infected with SARS-CoV-2, the virus that causes COVID-19, you may have had acute respiratory distress syndrome (ARDS). During the pandemic, 33% of people hospitalized with COVID-19 were reported to have ARDS.
Most people develop the syndrome after they’re hospitalized for a major illness such as sepsis or pneumonia or an injury to the lungs. Common signs of ARDS are severe shortness of breath, labored and unusually rapid breathing, low blood pressure, confusion, and extreme tiredness.
There is no cure or FDA-approved therapy for ARDS, which has a high rate of mortality. People with respiratory distress syndrome are usually put on ventilators to maintain oxygen and given steroids to reduce inflammation. But mechanical ventilation can exacerbate the initial injury and trigger more inflammation, leading to multisystem organ failure and death.
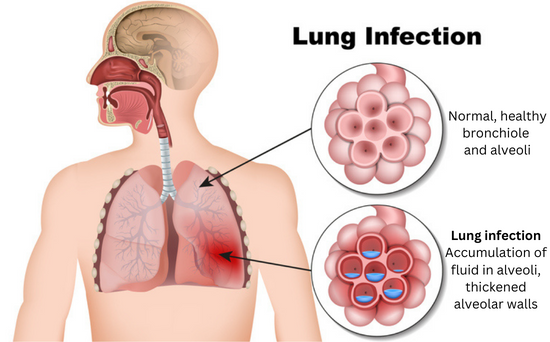
Inflammation plays a key role in developing ARDS. The immune system attempts to resolve the underlying infection or trauma by sending such a strong pro-inflammatory response that it creates too much inflammation. This causes fluid to build up in the tiny, elastic air sacs (alveoli) in your lungs, reducing the amount of air you breathe and sending less oxygen to your blood stream, which means less air reaches other organs.
But what if you could inhale a drop of solution designed to reduce that inflammatory response and allow your immune system to resolve the original infection?
Researchers at Ohio State University (OSU) have created a solution with engineered extracellular vesicles (EVs) that carry and release their anti-inflammatory cargo at the injured lung site. The solution was tested in mice with inflamed lung tissue that mimics ARDS. The results published in the journal Advanced Materials hint at the promise of a treatment for lungs severely injured by trauma or infection.
“To the best of our knowledge, this is the first study demonstrating that engineered extracellular vesicles derived from the skin can reduce key inflammatory mediators in a mouse model,” said lead investigator Natalia Higuita-Castro, Ph.D., associate professor of biomedical engineering and neurosurgery at OSU.
“The engineered EVs also changed the metabolic profile of cells to favor anti-inflammatory pathways, while significantly reducing tissue damage during acute lung injury,” she said.
Designing therapeutic nanocarriers
Higuita-Castro’s team chose extracellular vesicles derived from the skin cells of adult mice to engineer because they are naturally released in animals, including humans, and are natural nanocarriers. They can transport various active biomolecules, including proteins, nucleic acids, and antigens across biological barriers.
The next step was engineering the skin EVs to release a payload of specific proteins at the targeted site to treat the inflammation. The researchers loaded anti-inflammatory cytokines into the EV nanocarriers and tagged the surface with molecules that interact with specific receptor cells to improve their retention in the lungs, enabling targeted delivery.
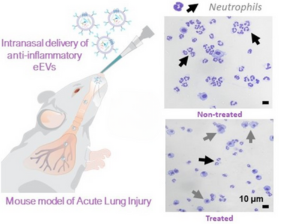
To evaluate the engineered EVs in mice, the animals were first given a drug that triggered major inflammation in their lungs. Then, some of the mice were given a single drop of liquid intranasally with the engineered nanocarriers that traveled directly to their injured lungs. Other mice remained untreated to provide a comparison.
Significant improvements
Credit: Paola Puerta, freelance artist
Within six hours of EV administration, the researchers saw a significant reduction in lung inflammation of the treated mice compared to untreated mice. This was measured by a reduction of neutrophils (a type of inflammatory cell) in the lungs of the treated mice. The reduction in inflammation was sustained up to 12 hours, according to co-first author Ana Salazar-Puerta, Ph.D., a postdoctoral researcher at OSU.
Another important finding was that the mice had significantly less lung tissue damage following treatment with the EVs. Compared with untreated mice, mice given EVs had a higher number of alveoli and the walls of the alveoli were thinner, which are both indications of improved lung function.
The scientists were surprised to see that the treated cells in the mice lungs also secreted substances with global therapeutic effects, including antioxidants and more anti-inflammatory molecules, according to their research.
"The results suggest that naturally derived nanocarriers are effective at reducing inflammation and tissue damage in the lungs of mice in a relatively short amount of time. If the findings are validated in future studies, this therapy could eventually benefit people with acute respiratory distress syndrome,” said Jermont Chen, Ph.D., a program director in the NIBIB Division of Discovery Science and Technology.
Future studies will need to use larger animal models to validate that this therapeutic approach can be scaled up, said Higuita-Castro.
She envisions broader therapeutic applications for engineered extracellular vesicles. “In the long-term, this could be an off-the-shelf product because these vesicles are highly immune-compatible and very versatile. Physicians may be able to use them for different conditions, including wound healing, diabetes, cardiovascular disease, and back pain,” she said.
This research was funded by grants from the National Institute on Aging and administered by NIBIB (DP2EB028110), the National Institute of Diabetes and Digestive and Kidney Diseases (DP1DK126199), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1R01AR079485-01A1), and The Ohio State University Office of Research.
This Science Highlight describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is foundational to advancing new and better ways to prevent, diagnose, and treat disease. Science is an unpredictable and incremental process—each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without the knowledge of fundamental basic research.
Study Reference: Salazar-Puerta AI, et al. Engineered Extracellular Vesicles Derived from Dermal Fibroblasts Attenuate Inflammation in a Murine Model of Acute Lung Injury. Advanced Materials (2023). DOI: 10.1002/adma.202210579
