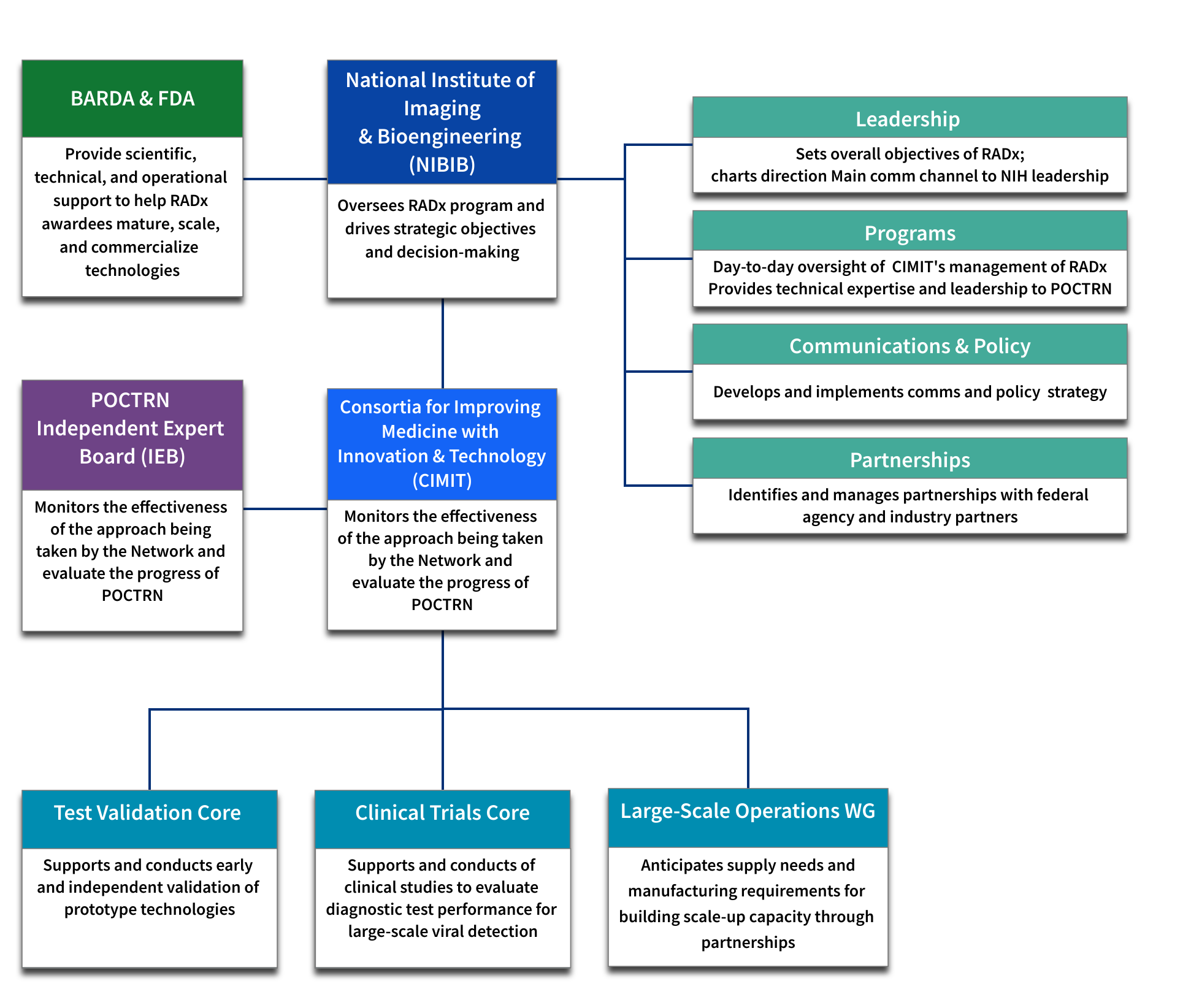
National Institute of Biomedical Imaging and Bioengineering - Oversees Rapid Acceleration of Diagnostics (RADx) Tech program and drives strategic objectives and decision-making.
- Leadership - Sets overall objectives of RADx Tech and charts direction, main communications channel to National Institutes of Health (NIH) leadership
- Programs - Day-to-day oversight of Consortia for Improving Medicine with Innovation and Technology’s (CIMIT) management of RADx Tech; provides technical expertise and leadership to the Point-of-care Technology Research Network (POCTRN).
- Communications and Policy - Develops and implements communications and policy strategy.
- Partnerships – Identifies and manages partnerships with federal agency and industry partners
Consortia for Improving Medicine with Innovation & Technology (CIMIT) - Monitors the effectiveness of the approach being taken by the Network and evaluates the progress of POCTRN.
- Test Validation Core - Supports and conducts early and independent validation of prototype technologies.
- Clinical Trials Core - Supports and conducts clinical studies to evaluate diagnostic test performance for large-scale viral detection.
- Deployment Core - Anticipates supply needs and manufacturing requirements for building scale-up capacity through partnerships.
POCTRN Independent Expert Board (IEB) – Monitors the effectiveness of the approach being taken by the Network and evaluates the progress of POCTRN.
Biomedical Advanced Research and Development Authority (BARDA) and Food and Drug Administration (FDA) - Provide scientific, technical, and operational support to help RADx awardees mature, scale, and commercialize technologies.
