Collaborators
Project Brief
Analytical ultracentrifugation, though a classical biophysical discipline, has undergone a renaissance in the last decade due to new computational capabilities and new instrumentation, with increasing applications in structural biology and immunology, for example, for the study of protein interactions and multi-protein complexes, and in biotechnology industry for the characterization of protein pharmaceuticals and nanoparticles for drug delivery.
Very recently the Section of the Dynamics of Macromolecular Assembly (DMAS) has embarked on the use of fluorescence detection in analytical ultracentrifugation (fdAUC). Using a commercial fluorescence detector, in combination with newly developed computational tools, DMAS has achieved unprecedented sensitivity, which allows the characterization of protein assemblies in the low picomolar concentration range and from unpurified cell extracts. This has great potential to serve as a complementary tool to single-molecule fluorescence microscopy by measuring the size of macromolecular complexes in fdAUC after their observed co-localization determined by cellular imaging. Unfortunately, the commercial fluorescence detector is limited to a single excitation wavelength and allows only very constrained sedimentation and detection modes.
Jointly DMAS and SPIS want to bring to bear for fdAUC the entire toolset of fluorescence spectroscopy with the following goals:
- Detection of protein complex formation and size determination of weakly associated reversible macromolecular assemblies in crude cellular extracts of macromolecules with 105 or fewer copies per cell.
- Integration of optical detection models with new centrifugal sedimentation modes suitable for large assemblies and nanoparticles.
- Multi-channel detection of several fluorophores to improve specificity in the study of multi-protein interactions.
- Flexible excitation and emission wavelength selection to detect energy transfer and minimize background from cellular autofluorescence.
- Pulsed excitation and time-gated detection for rotational diffusion measurements to enhance macromolecular size and shape measurement.
- To achieve these goals, it will be essential to design a novel, flexible ultracentrifugal fluorescence detection platform capable of accommodating high-speed multi-channel excitation and emission detection, controlling switchable polarizers and filters, and detecting time-resolved fluorescence.
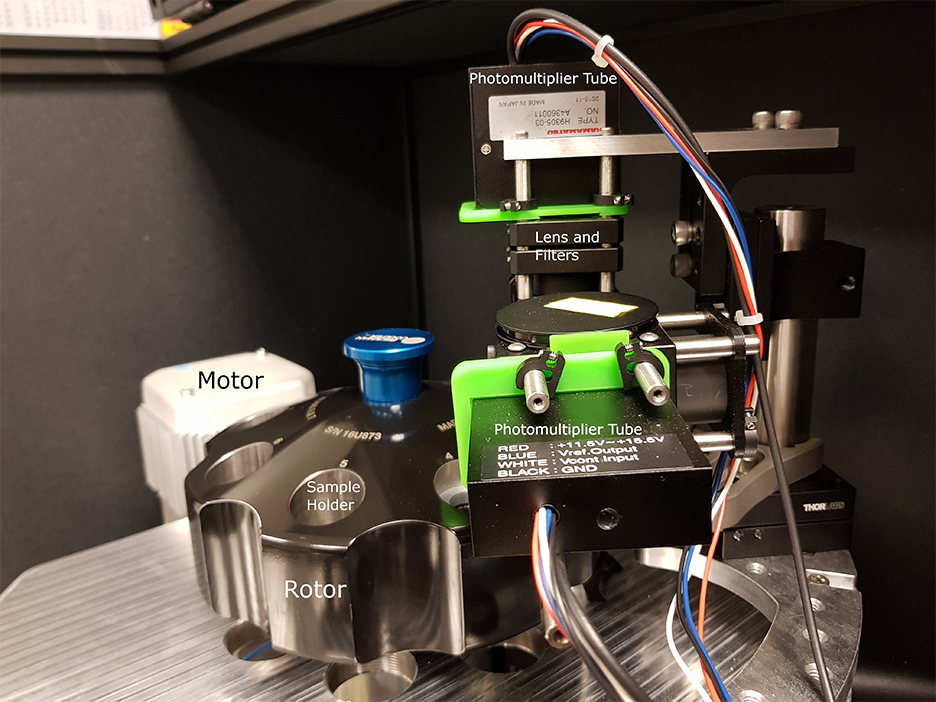
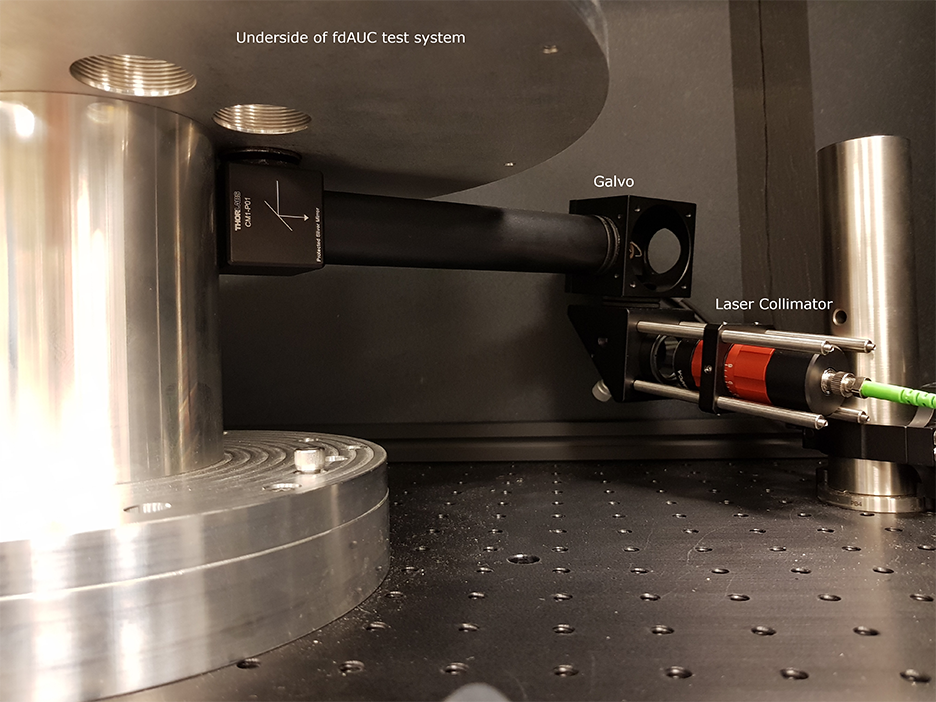
To this end, DMAS has already modified the commercial analytical ultracentrifuge to equip the ultracentrifugal high vacuum chamber with multiple optical and electrical feedthroughs and an optical table localized close to the spinning rotor. For the next stage, we are in the process of building a mock-up centrifuge to experiment with the optical design and test data acquisition and signal processing modes.
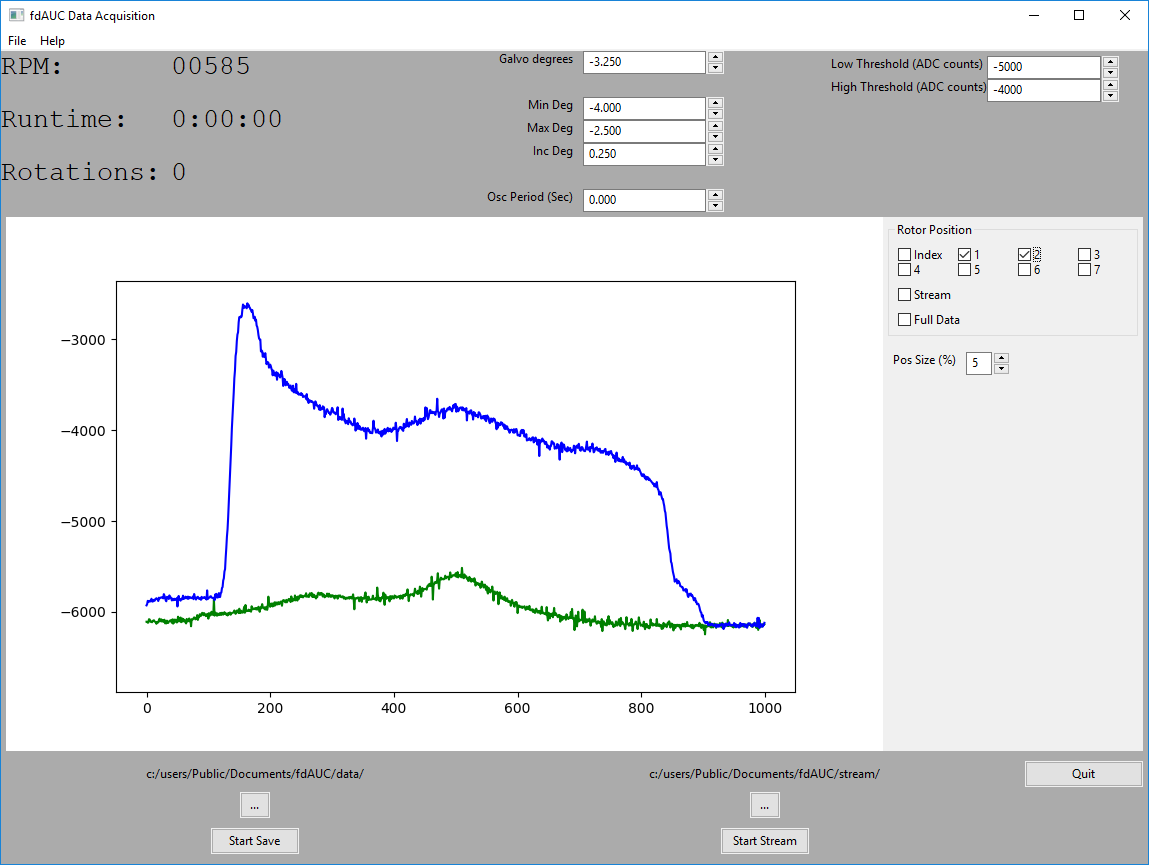
The design of fdAUC with real-time signal processing requires measurement and synchronization with sample rotation in the microsecond time-scale, sample pre-processing across the nanosecond time scale, control of optical elements for radial scanning in the time-scale of seconds, and storage of data for further analysis across a time-scale of hours. To achieve these goals, SPIS specified and installed a commercial data acquisition system to integrate with the photomultiplier tubes (PMT) used to measure the fluorescent signals. In addition, SPIS designed and implemented custom PMT amplifier circuitry to achieve the required amplification and bandwidth. Finally, SPIS wrote custom application software providing an interface for the user to control the system parameters, process the data in real-time, and display the final results. The instrumentation and algorithms accomplishing these tasks will form the backbone for the implementation of the different fdAUC modes envisioned above.
We anticipate multiple milestones in this project, each expanding our capability in macromolecular characterization. The first – likely achievable even with the mock-up centrifuge – will be a stationary AUC detector for rapid-sedimenting and non-diffusing particles, which will greatly extend the size-range for AUC analysis. After installation of the fluorescence detection platform in the ultracentrifuge, we anticipate multi-spectral detection in standard sedimentation velocity modes will increase the complexity of interacting protein systems that can be studied. DMAS has several NIH IRP collaborative projects studying molecules from different immunological pathways where these new capabilities could be tested and immediately exploited, while ongoing fdAUC development work could optimize sensitivity and explore innovative strategies.