Collaborators
Project Brief
Patients cite pain as the most common reason for seeking health care, and some medical organizations include pain as a fifth vital sign along with blood pressure, heart rate, temperature, and respirations. While the understanding of the neurophysiologic mechanisms by which noxious and non-noxious stimuli are perceived has substantially improved, the mechanisms of pain are not entirely understood. Despite progress in the development of new drugs and treatments, humans still suffer a great deal of pain while in the hospital, with many suffering from debilitating chronic pain throughout their lives.
Using humans in pain research has many limitations, including the lack of objective measures of pain and the inability to control environment factors. Our goal was to establish a model of non-injurious pain in mice which would overcome these limitations. Mouse models allow an investigator to study specific pain mechanisms by using transgenic animals, controlled environmental conditions, and larger sample sizes. As a key component of this model, we used an FDA-approved neuro-stimulator that is currently used in humans (adult and children) for the diagnosis of pain syndromes and neurologic diseases. The neuro-stimulator delivers electrical stimuli to the skin at different frequencies and intensities producing transient discomfort without injury. During a pilot study, we demonstrated that mice vocalize at repeatable thresholds of stimulus, providing an objective measure of pain. As currently used pain models for mice may produce some tissue-injury to induce pain, the development of a non-injury producing animal model adds an invaluable tool for the study of the mechanisms of pain and the understanding of the transmission of pain.
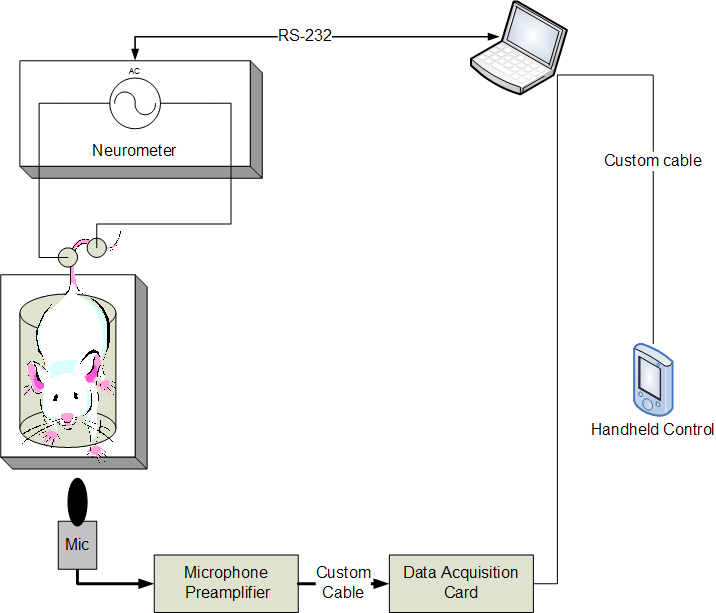
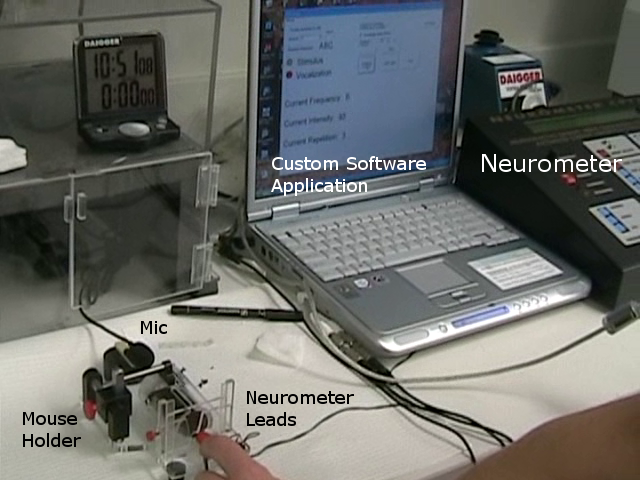
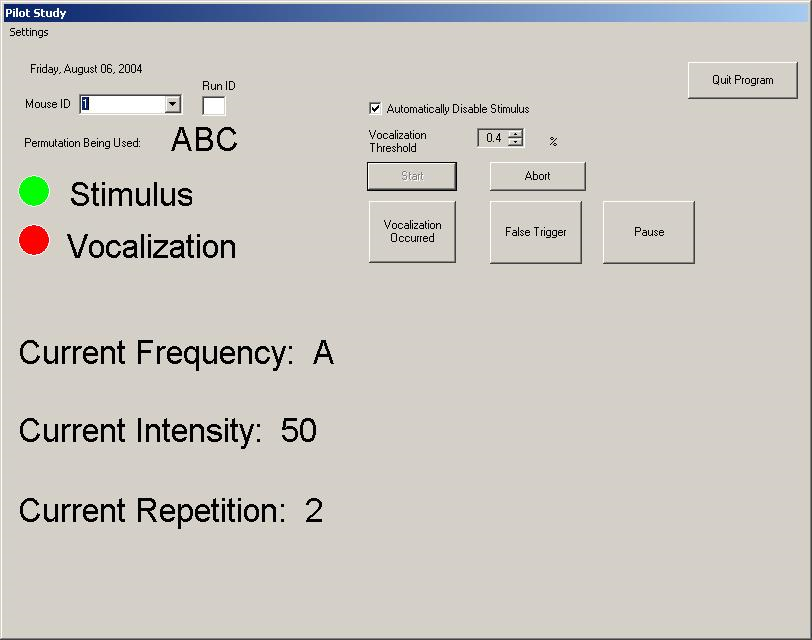
IDEAS engineers integrated the commercially available neuro-stimulator device with a microphone, laptop, remote handheld control, and custom software application. The custom software application automates the delivery of the electrical stimulus per pre-defined protocols, reducing investigator fatigue relative to manually implementing lengthy, repetitive stimulus protocols. In addition, the application analyzes real-time audio signals from the microphone to automatically detect a mouse vocalization in presence of noise caused by the movement of the mouse and nearby environment noise sources. The detection of a vocalization causes immediate cessation of the stimulus, preventing unnecessary discomfort to the mouse. The remote handheld device allows the investigator to manually halt the stimulus in the case of a missed vocalization, or abort the experiment due to other animal safety concerns or general experiment difficulties.
Tech Transfer:
Software Licensing: Neurotron, Inc
