Collaborators
Project Brief
NIH researchers introduced an innovative technique for high density arraying of archival clinical tissue in the research and clinical laboratory. Consisting of an array of cylindrical cores extracted from formalin-fixed paraffin embedded tissue samples, tissue microarrays (TMA) have become widely used as a powerful validation tool for high throughput genomic screens. TMAs allow a researcher to quickly compare many different tumor types, a single tumor type over multiple patients, or tumor progression on a single pathology slide; significantly reducing the costs associated with reagents and processing, as well as improving analysis reproducibility. The original manual process uses a hollow needle to remove cores from standard pathology blocks (i.e., donor block) that are then deposited into a pre-made hole in a block of paraffin (i.e., recipient block). When completed, the recipient blocks are sectioned to create pathology slides for downstream analysis. While successful, the manual construction of the TMAs is time consuming and requires training to produce quality arrays.
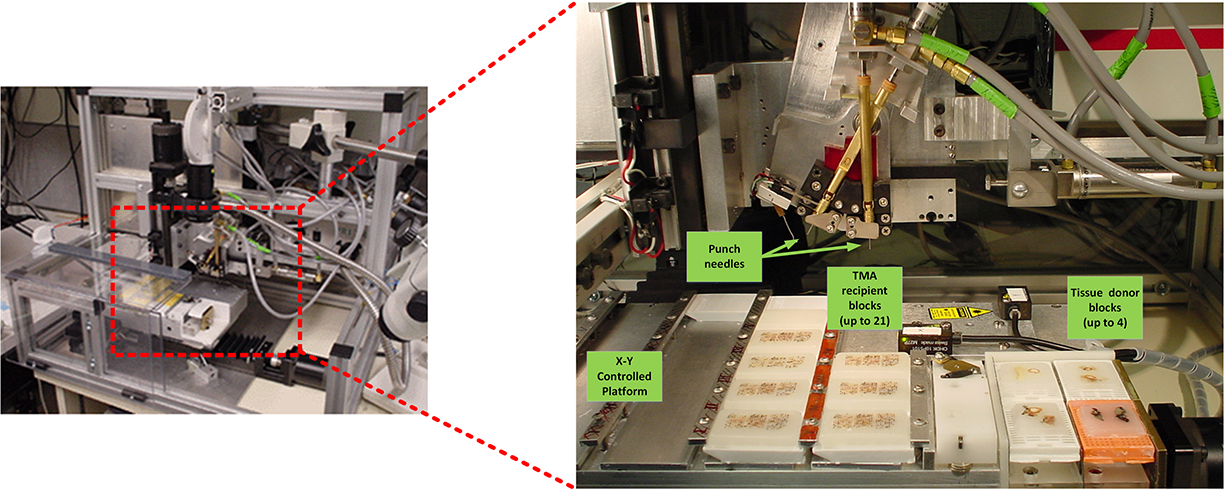
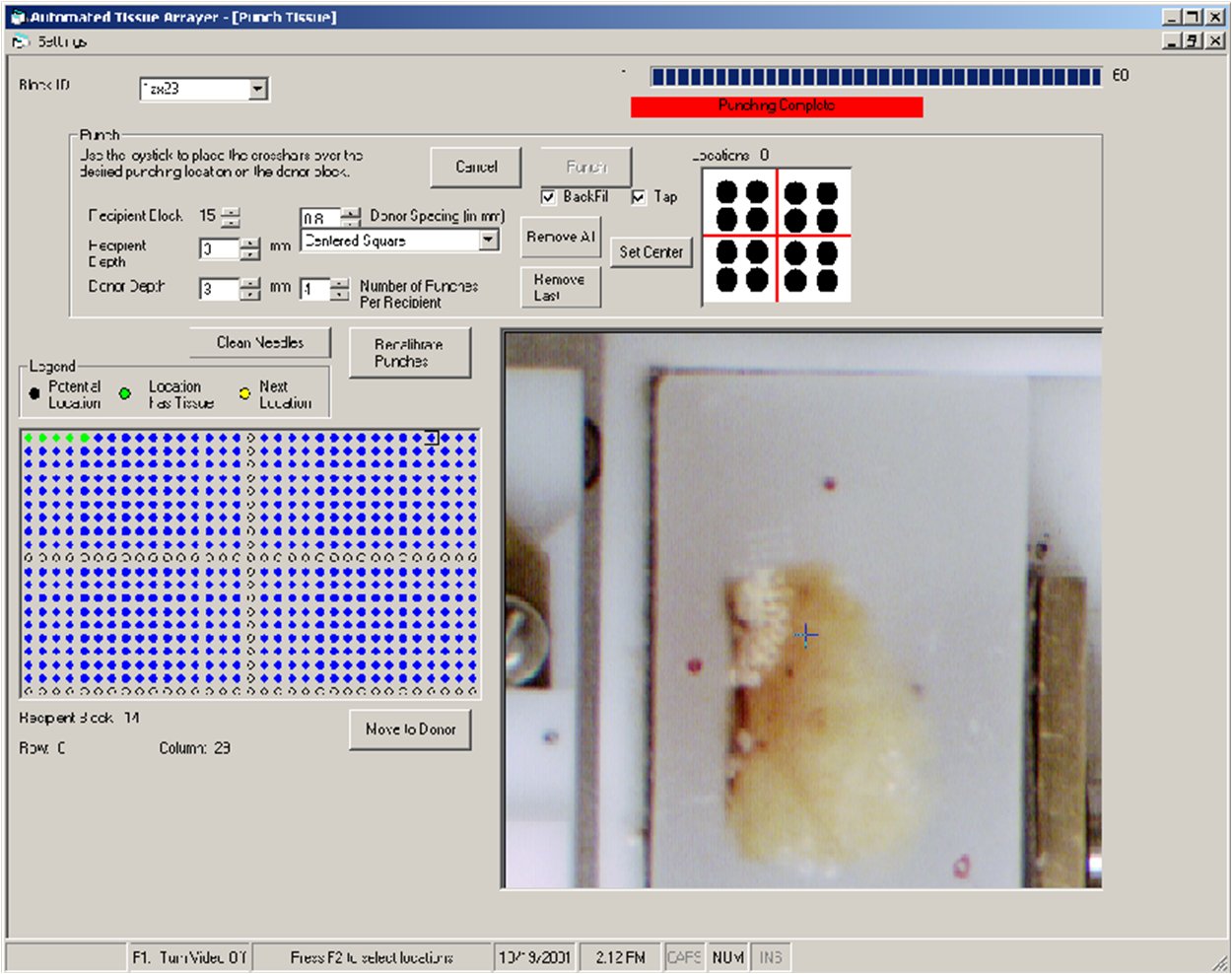
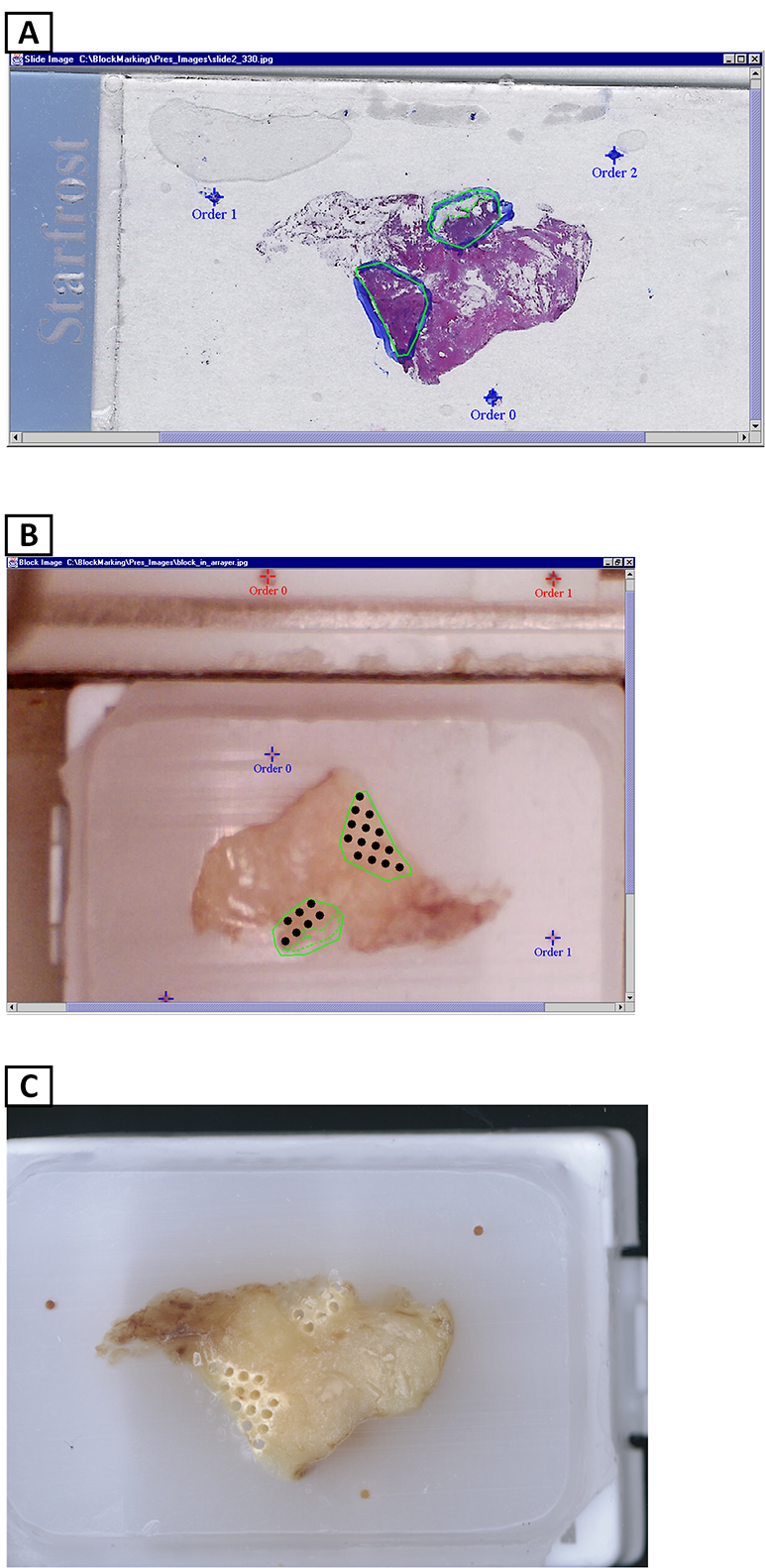
In collaboration with NHGRI researchers and a commercial company, we designed and fabricated a robotic arrayer to semi-automate the TMA construction procedure. The custom arraying apparatus, enabling automatic tissue core acquisition and deposition with X-Y-Z controls, provides a platform capacity for 21 recipient blocks and 4 donor blocks for large scale TMA production. To achieve higher specificity in choosing donor block tissue, a macroscopic method was employed to localize and mark desired targets in the donor block. Specifically, the paraffin block sample sites are marked with an overlay of the corresponding glass slide H&E section over the transluminated donor block. While successful, this method can lead to inconsistently sampled microscopic lesions and non-informative TMA tissue section spots if the target area is not carefully chosen.
Using the semi-automated arrayer, collaborators in NHGRI developed a TMA which led to an important finding for BRAF mutations in nevi.
Tech Transfer
Employee Invention Report. 2004. Tissue Microarray Technology. Method and system for processing regions of interest for objects comprising biological material. NHGRI, CIT.
Employee Invention Report. 2003. Tissue Microarray Technology. High-throughput tissue microarray technology and applications. NHGRI, CIT, Beecher Instruments Inc.
