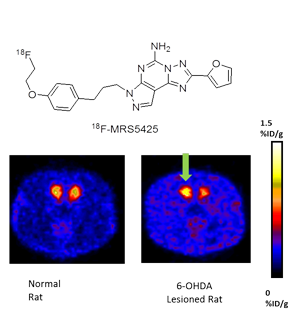
Small Molecule Probes
There are numerous targets for drug-like molecules that are of interest due to their role in human disease. Imaging studies can be utilized as a research tool to help determine optimum drug dosing and to elucidate biochemical function. In addition, imaging can be used for diagnosis and staging of disease and for monitoring treatment response. We have developed methods for the incorporation of fluorine-18 and bromine-76 into small molecule tracers with applications for studies of brain function and disease and for diagnosis, staging, and treatment management in oncology. Adenosine A2a and A3 receptors, corticotrophin releasing hormone (CRH) ligands, muscarinic, and serotonin ligands, CXCR4 are a few examples.
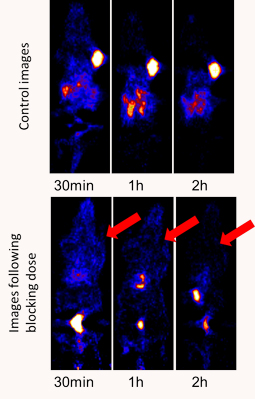
Targeting Employing Proteins and Peptides
Antibodies are specific molecules that bind to a particular antigen. Imaging of radiolabeled antibodies has been investigated for many years. The challenge with antibody imaging is the long half-life in the blood. One approach is to radiolabel with longer lived radionuclides. The non-traditional PET radioisotopes such as Br-76, Y-86, Cu-64, Zr-89, and I-124 and SPECT radionuclides such as I-123 and In-111 offer longer half-lives that may be more useful for this application.
An alternative approach is to develop smaller proteins or peptides that display high binding specificity but have more rapid pharmacokinetics. Peptides and proteins can be functionalized first in order to allow site-specific labeling with radiohalides or radiometals as well as fluorophores. We have conducted conjugation reaction of peptides and proteins with various macrocyclic chelators to allow radiolabeling with radiometals such as Cu-64, Ga-68, and Zr-89. We have developed or adapted from the literature small organic prosthetic groups labeled with fluorine-18 for conjugation with proteins and peptides.
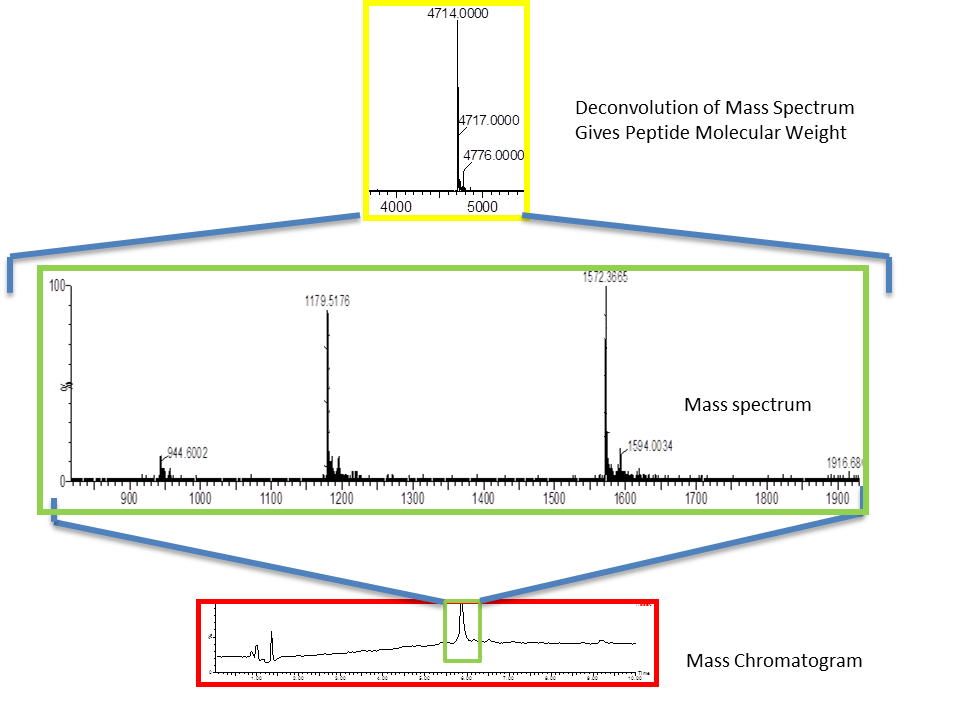
Analytical Techniques
We have expertise in the use of highly sensitive, high resolution mass spectrometry coupled with HPLC (LC-MS) to confirm the identity of all of our tracers, from small molecules up to small proteins and oligonucleotides. In addition, we have experience using LC-MS to assist in the identification of metabolites. The results allow a qualitative assessment of the propensity of radiolabeled metabolites to interfere with interpretation of images, an assessment of species differences anticipated as the compounds move toward eventual clinical use, and can assist in the preparation of improved ligands by modification of chemical structure to alter the metabolism. The mass spectrometry results have been utilized to assist in the development and validation of thin-layer chromatography (TLC) and/or extraction methods to provide parent corrected plasma input functions for PET modeling. The high quantitative sensitivity of the mass spectrometer for some chemical structures can allow determination of biodistribution without the need to synthesize the radiolabeled compound. The sensitivity is sufficient to quantify the membrane binding and cellular internalization of ligand molecules. We have extended this technology to study metabolism pathways of radiolabeled and fluorescently labeled peptides and proteins.