Chemical cage is designed to shuttle probe to low-oxygen targets
Hypoxia, a lack of oxygen, is associated with numerous diseases including cancer, heart disease, arthritis, and diabetes. Certain tumors grow very rapidly in hypoxic environments and the ability to identify such tumors could be extremely useful for determining the best course of treatment. Using non-invasive magnetic resonance imaging (MRI) would be an ideal way to image such tumors but is a challenge because the standard contrast agents do not work well in hypoxic tissues with few blood vessels. Now researchers funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) are developing new MRI contrast agents that are activated in low oxygen environments enabling improved diagnosis and treatment of hypoxic tumors as well as other diseases that have a hypoxic component.
For over 30 years contrast agents such as gadolinium have been used to obtain enhanced MRI images. The agents are made from a group of elements known as the rare-earth metals that have unique magnetic properties.
Chemists at Wayne State University in collaboration with researchers at Baylor College of Medicine are using another rare-earth metal—europium (Eu)—to obtain enhanced MRI images of areas of hypoxic disease in the body. The team, led by Matthew J. Allen, Ph.D. professor and chair of the department of chemistry, have been working with Eu because it has the distinct property of creating a bright MRI signal in response to low oxygen levels.
The idea of using Eu as an MRI-enhancing agent, however, comes with an interesting twist. Although it gives a bright MRI signal in low-oxygen environments, if it contacts normal, higher oxygen levels it is inactivated. Because MRI contrast agents need to be shuttled through the circulatory system—in the highly oxygenated blood—Eu would be inactivated before reaching a low-oxygen target such as a tumor that grows rapidly in a low-oxygen environment.
“This is the type of problem biomedical engineers like to try to solve,” explains Tatjana Atanasijevic, PhD., manager of the program in molecular probes and imaging agents at NIBIB, which funded the work. “The Wayne State team is well suited to engineering a biochemical solution to shuttling Eu through the circulation to target tissues.”
Allen’s team set out to design a protective “chemical cage” that would surround the Eu in the circulation—fending off the oxygen, which has a relentless chemical “need” to interact with such compounds, and, in this case change the Eu structure to the irreversible inactive form.
They designed and synthesized a chemical library of 20 compounds with subtle variations in their structure which caused them to bind in chemically different ways to the Eu.
The Eu compounds were tested using MRI when added to an oxygen-rich solution. The compounds that were rapidly deactivated by oxygen would basically go dark on the MRI. However, one of the compounds, designated Eu-5 maintained the baseline MRI “glow” that is seen when the Eu has not been deactivated.
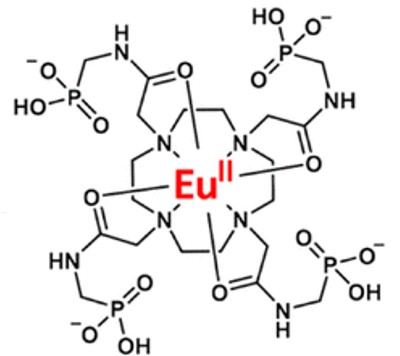
“The Eu-5 compound successfully fended-off the attacking oxygen in the solution,” explained Allen. “The MRI signal coming from Eu-5 gives us a clear indication that the chemical cage is successfully protecting Eu from being deactivated by oxygen.”
To test its effectiveness, researchers injected Eu-5 into the tail vein of a mouse where it was challenged to survive in the oxygen-rich blood. An MRI of the mouse after several minutes in the high oxygen environment indicated that the Eu remained intact—maintaining a persistent signal under MRI in the blood vessels. This result contrasted with the non-protective compounds that essentially went dark in the mouse experiment due to oxygen inactivation.
“Because it is so reactive, oxygen will inevitably break through chemical structures designed to keep it at bay,” said Allen. “What Eu-5 has accomplished is dramatically slowing the process, which would enable the compound to move through the blood to arrive at hypoxic tissue targets making it useful as an MRI probe to identify and monitor regions of low oxygen in tissues.”
The group is now in the process of more extensive testing of Eu-5 in mouse models of disease. A first test of Eu-5 will be its ability to travel successfully through the circulatory system of a mouse cancer experimental model and arrive at and enhance the MRI of hypoxic tumors. Because hypoxia in solid tumors is associated with poor prognosis and resistance to certain therapeutics, Eu-5 enhanced MRI images of hypoxic tumors could be an extremely valuable clinical tool for improved cancer treatment.
The work was reported in the Journal of the American Chemical Society1. This research was supported by grant R01EB027103 from the National Institute of Biomedical Imaging and Bioengineering, the National Cancer Institute, and the National Institute of General Medical Sciences. The authors acknowledge the use of the 9.4T MRI at the Small Animal Imaging Facility at Texas Children’s Hospital.
-Written by Thomas Johnson, Ph.D.
