Case study demonstrates feasibility of device, uses wearer’s residual muscles for postural stability
Prosthetics can greatly improve an amputee's quality of life, yet current lower-limb devices can't provide continuous neural control of balance or posture, which can lead to a variety of consequences, such as difficulty walking on certain surfaces or an increased likelihood to fall. Now, NIBIB-funded researchers are working on an ankle prosthetic that relies on the user's residual muscles—and the electrical signals that they generate—to help amputees with their postural control.
Roughly 2 million people living in the United States have had an amputation, with approximately 185,000 amputations being performed in this country every year.[1] Amputations of the lower limbs are the most common, and many individuals will choose to use a prosthetic device to assist in walking and other types of ambulation. The majority of lower-limb prostheses are passive devices, which are designed to store and return energy during walking, but do not provide power or allow a normal range of motion. Meanwhile, most powered prostheses can help the user to move their residual limb, but require external sensors to help anticipate the user’s movement. This prototype ankle prosthetic, on the other hand, is controlled by directly mapping the electrical activity generated from the user’s muscles without the need for external sensors or complex automation. The neurally controlled, powered prosthetic device relies on training the user’s residual muscles to create continuous control of their posture and balance.
“This study is a culmination of work demonstrating that a prosthetic user can be trained to provide continuous neural control during various postural tasks,” said Grace Peng, Ph.D., director of the NIBIB program in Mathematical Modeling, Simulation and Analysis. “This case study is an important first step in implementing this unique control framework for future ‘user-driven’ prosthetic devices.”
To use a joint, either consciously or subconsciously, our brain sends an electrical signal to the necessary muscle groups, resulting in the contraction of muscle fibers and enabling movement. After amputation, the muscles in the residual limb can still receive these electrical signals, explained Helen Huang, Ph.D., a professor in biomedical engineering at both North Carolina (NC) State University and University of North Carolina (UNC) at Chapel Hill. Her research group, hoping to harness that signaling ability, is working to develop a lower-limb prosthetic device operated solely by the body’s own electrical signals, a concept known as direct electromyographic (or dEMG) control. Huang and her team recently reported their case study evaluating this dEMG-controlled prosthetic, operated by an individual with a transtibial amputation (where the limb is removed below the knee), in Wearable Technologies.[2]
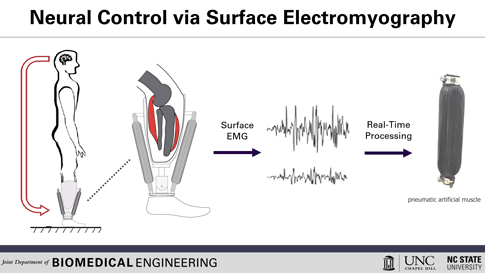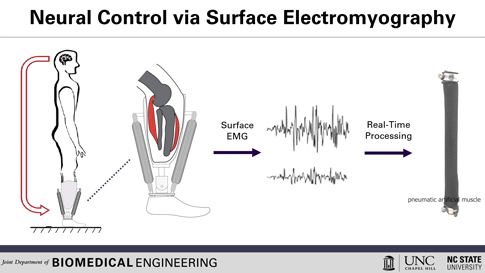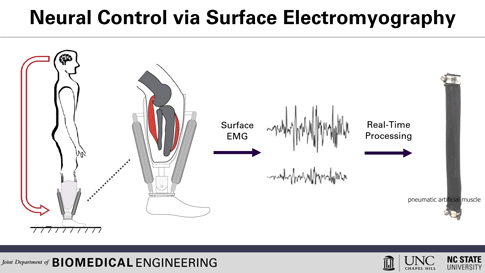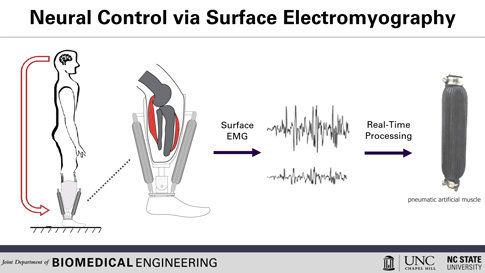
Here’s how the prosthetic device works: Surface electrodes, which can detect the electrical signals in the user’s residual muscles, are placed on the residual limb. When the user contracts their residual muscles, with the intention to flex their foot, for example, the electrodes collect and process the associated EMG activity. This EMG signal drives pneumatic artificial muscles, which function using pressurized air to contract or extend, allowing the user to continuously control the movement of the artificial limb.
“Often times, if part of our limb is removed, we start to use the muscles in the residual limb a little bit differently,” explained Huang. This can result in altered muscle contraction patterns, and when a dEMG prosthetic device is used, the intended limb motion and the actual limb motion will be out of sync, she said. For this reason, the prosthetic user, with the aid of a physical therapist, “trains” their residual muscles to better operate the device. The training, which lasted three weeks in this case study, included daily life activities that require postural control, such as transitioning from sitting to standing, picking up a heavy object, or reaching forward. “This regimen helps the user to train their muscles—and their brain—to better control the prosthetic ankle,” explained Aaron Fleming, a graduate student in the Huang lab and first author of this case study.
After the training, Huang and colleagues evaluated the participant’s stability while he was performing specific tasks, either with his usual passive prosthetic ankle, or with the new dEMG-controlled device. His stability was markedly improved when wearing the dEMG-controlled device, even when standing on a foam surface (which requires additional postural control) or with his eyes closed (which also tests balance). The researchers also looked at the synchronization between his intact limb and his artificial limb and found that it was far higher when he was wearing the dEMG-controlled prosthetic compared with his usual passive device, both when he was standing or when he was picking up a heavy load.
“Postural stability is something that many able-bodied people take for granted,” Huang said. “Being able to stand in a crowded space without being afraid that you’ll fall if someone bumps into you—this is one of the many challenges that people with lower-limb loss face,” she said. “While this research captures just one case study, it demonstrates the feasibility of developing a device that allows the user to intuitively adjust their posture, which could greatly increase their quality of life.”
Huang mentioned that they are currently investigating the use of their prosthetic ankle among several other participants with lower-limb loss, and that they will evaluate additional tasks beyond those in this case study—including walking.
This work was supported by grant EB024570 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB); by grant F31HD101285 from the National Institute of Child Health and Human Development (NICHD); and by the National Science Foundation (NSF).
[1] Lower limb loss statistics provided by the Amputee Coalition.
[2] Aaron Fleming, Stephanie Huang, Elizabeth Buxton, Frank Hodges, and He Helen Huang. “Direct continuous electromyographic control of a powered prosthetic ankle for improved postural control after guided physical training: A case study.” Wearable Technologies (2021), 2, e3. DOI:10.1017/wtc.2021.2
