Researchers shrink electrophoresis technology to fit on chip
Researchers funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), part of the National Institutes of Health, have developed a new 3D printed device that will make it faster and easier to identify women at risk for premature birth. Children born before 37 weeks are considered preterm and are more likely to suffer from severe health complications in the lungs, heart, and brain. Worldwide, approximately 1 million babies die each year due to the consequences of preterm birth. Ideally, doctors would like to know which women are most at risk for preterm birth so they can more carefully monitor those patients. However, current screening technology requires sterile lab conditions. This makes it impossible to screen women in low resource settings where that information could be the most helpful.
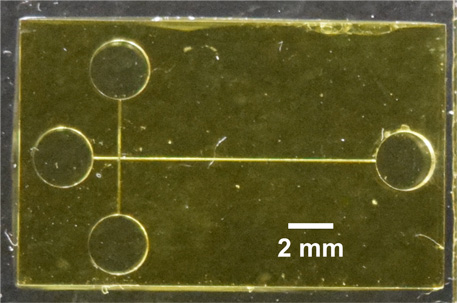
Researchers at Brigham Young University have developed a new way to screen for previously identified protein and peptide biomarkers that indicate a risk for preterm birth. Professor Adam Woolley, Ph.D., and his team have been able to create an electrophoresis chip. Electrophoresis is a process that separates biological material such as proteins and DNA based on size, using electrical current. In the lab, researchers often use a gel, which acts as a microscopic net that biological material has to wiggle through. Larger materials are left behind, while smaller materials are able to move more quickly through the gel. The material is labelled with fluorescent molecules, and since researchers know the size of the biomarkers they are looking for, they can identify samples that contain the biomarkers that indicate a risk of preterm birth.
Unfortunately, this technique requires a sterile lab, a special camera, and many hours. Woolley and his team have recreated a similar process on a small chip that can be used anywhere—even outside a lab. Instead of a gel, the team 3D printed small channels onto the chip, allowing separation to occur without the use of a gel.
“Our 3D printing technology has the potential to revolutionize the creation and usage of miniaturized diagnostic devices," said Woolley. While 3D printed devices have been used in other diagnostic areas, this is the first time the channels have been printed at such a small scale, 50 micrometers—smaller than the width of a human hair. They achieved this by developing two new techniques. First, they created a unique resin that they used as ink in the 3D printer. Second, they used careful control of light exposure on voxels at the edge of the printed channels to better control their height and width. This made it possible to separate biological materials of similar size on a chip for the first time.
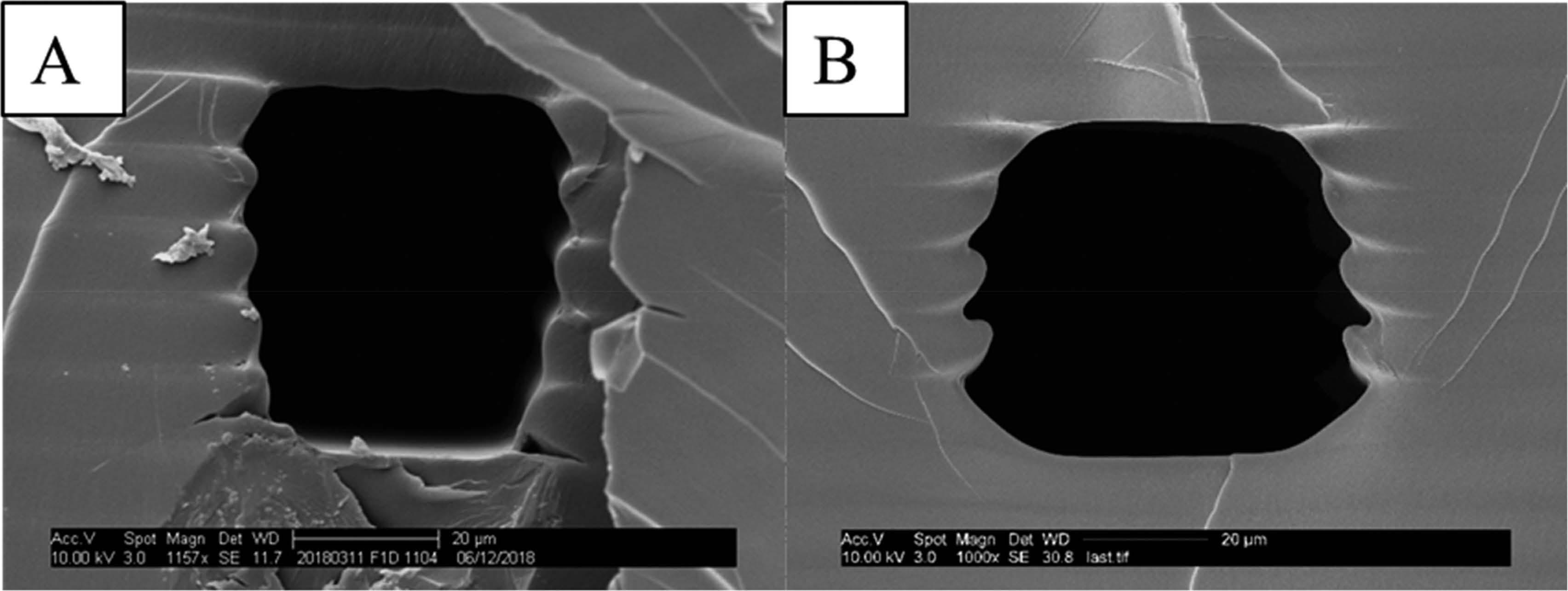
“This technology is an excellent example of how microfluidics can open the door to take protein screening out of the lab, making it more accessible in low-resource settings and around the world,” said Seila Selimovic, Ph.D., program director for Engineered Tissues at NIBIB. “These electrophoresis chips could someday be a point-of-care diagnostic for other small molecular biomarkers for diseases such as Alzheimer’s and Parkinson’s.”
3D Printed Microfluidic Devices for Microchip Electrophoresis of Preterm Birth Biomarkers. Beauchamp, M. J., Nielsen, A. V., Gong, H., Nordin, G. P., & Woolley, A. T. (2019). Analytical Chemistry, 91(11), 7418-7425. doi:10.1021/acs.analchem.9b01395
