Bioengineers have developed a 3D printing technique that creates the interacting networks for transport of air, blood, and other bodily fluids—a major step toward 3D printed replacement organs.
In the field of tissue engineering, a major hurdle towards creating lasting functional tissues has been the difficulty of printing the complex networks of blood vessels, airways, and other systems that can reliably supply the tissue with the necessary nutrients, and clear away waste products.
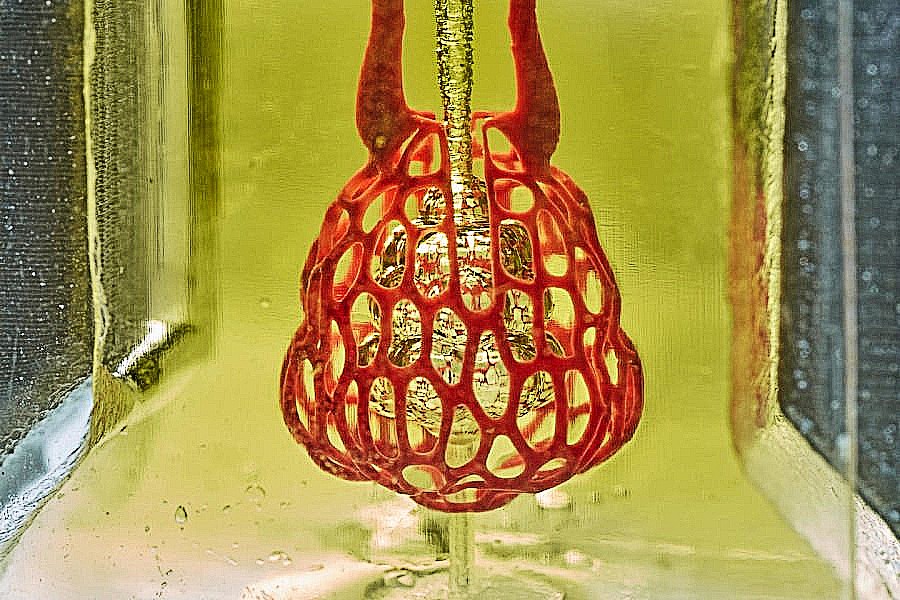
Now bioengineers led by Jordan Miller, Ph.D., Assistant Professor of Bioengineering at Rice University, and Kelly R. Stevens, Ph.D., Assistant Professor of Bioengineering and Pathology at the University of Washington, have developed a novel 3D printing technique that is a significant breakthrough in the push to provide replacement organs. The team included 15 collaborators from Rice, UW, Duke University, Rowan University, and Nervous System, a company in Somerville, Massachusetts. The results are reported in the May issue of Science.1
“It is not surprising that it took a large interdisciplinary team including bioengineers, tissue engineers, pathologists, and computational scientists to devise the unique approach necessary to build the intricate system needed to deliver nutrients to the bioprinted tissues,” said Seila Selimovic, Director of the NIBIB Program in Tissue Engineering, which partially funded the study. “It will be exciting to see how this game-changing breakthrough plays out in terms of our ability to bioprint and transplant working organs.”

The approach added a unique level of precision and detail in 3D bioprinting that had not previously been possible. The group used a technique called stereolithographic printing, which is most prominently used with resins that harden into the desired pattern when hit with a laser. However, hard resins could not support living cells. By adding laser sensitive food dyes to the biogel material necessary to support living cells, the team was able to extend levels of printing complexity and scale achieved previously only with resins, to biogels. The added dyes were the key to laying down and solidifying many consecutive thin layers of biogel with the intricate vascular patterns of an organ.
The team printed a number of organ-like structures, including the microstructure of the lung where vessels carrying air and blood wrap around each other. This is the interaction in the lungs that allows the air we breathe to be transferred to the bloodstream and delivered throughout the body. In tests under the pressures found in the human lung, the lung-mimicking structure was able to expand with the inflow of oxygen and the net-like structure of blood vessels surrounding the “breathing” air sac was able to take up the oxygen.
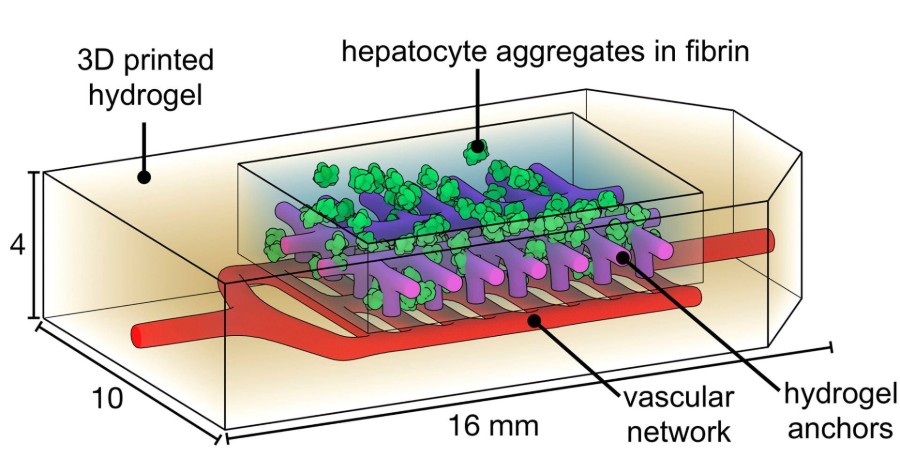
The team also printed liver-like structures, which survived when implanted into mice with liver injury, as well as blood vessels with working valves that mimic the valves controlling the movement of blood and other fluids in the heart, leg veins, and lymphatic system.
“The design of this system is an important step towards tissue engineering organs such as liver, which is an extremely complex organ with many functions,” explains Dr. Stevens. “The ultimate goal would be to someday be able to provide bioprinted livers to thousands of individuals on transplant waiting lists.”
Although it is a lofty goal, the team believes that the progress they continue to make could make organ bioprinting a major component of medicine within the next twenty years.
The work was supported by the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation, the John H. Tietze Foundation, the National Science Foundation (1728239, 1450681 and 1250104), the National Institutes of Health (F31HL134295, DP2HL137188, T32EB001650, T32GM095421 and DP5OD019876) and the Gulf Coast Consortia.
