Tracking medications, finding tumors easier with new technique
A study conducted by NIBIB-funded researchers and published in Science describes a new method to create radioactive tracers that are used for medical imaging, specifically positron emission tomography (PET). The method allows them to attach radioactive atoms to compounds that have previously been difficult or even impossible to label. The advance will make it easier to track medications in the body and identify tumors and other diseases.
PET imaging is a powerful tool used by doctors to make medical diagnoses, and more recently, a technology used by researchers in drug discovery to track drugs as they travel through the body. “This method describes a new way to attach a radioactive atom to a variety of compounds, including medications, and could potentially create a whole new class of radiotracers,” says Tatjana Atanasijevic, Ph.D., manager of the NIBIB program in Molecular Probes and Imaging Agents.
PET scans use radiotracers, which are made up of a radioactive atom that is attached to a specific molecule, which varies depending on the purpose of the scan. The tracers bind to the targets in organs or tissues following injection or ingestion into the body. Then a PET scanner is used to help doctors visualize and quantify the amount of the tracer and thereby observe changes or disease in the body.
Fluorine-18 is one of the most commonly used radioisotopes used for PET imaging. Still, researchers have been limited in tagging a broad spectrum of compounds with Flourine-18 because it is challenging to attach small molecule drugs to the compound. A team of researchers at the University of North Carolina (UNC) at Chapel Hill have described a new method to convert a carbon and hydrogen bond to a carbon and fluorine bond that can be used to generate new radiotracers.
David Nicewicz, Ph.D., a professor in the UNC Department of Chemistry, has been developing a method to make specific chemical structures, called aromatics, more reactive using simple organic dyes that are activated by blue light from a laser.
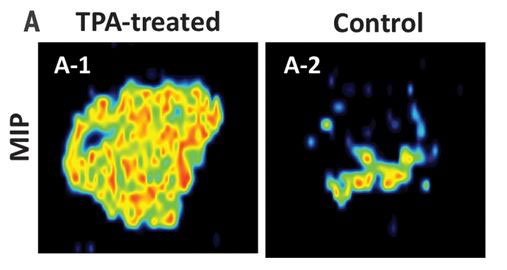
Nicewicz and Zibo Li, Ph.D., a professor in the UNC School of Medicine Department of Radiology, teamed up to introduce Fluorine-18 into drug molecules that contain an aromatic structure. The group successfully tested the method on several different aromatic compounds and demonstrated that tumors in mice could be imaged using the new radiotracers. “Since we can use existing drugs with the aromatic structure, the applications of these agents are broad. I can see this method being used to easily and quickly generate new agents for cardiovascular, neuroscience, and cancer research,” said Li.
The researchers are working on expanding their method to create new radiotracers and have early indications that the same chemistry will work with another radioactive atom, Carbon-11. “Our team has taken a basic science project and transformed it into applied science that may be used daily in research and hospitals in the future. It’s exhilarating to see the intersection of chemistry and biology in ways I could not imagine on my own,” said Nicewicz.
This research was supported, in part, by National Institutes of Health grants from NIGMS (GM120186) and NIBIB (EB014354).
Chen, Wei, et al. “Direct arene C-H fluorination with 18F-via organic photredox catalysis.” Science, American Association for the Advancement of Science, 21 Jun. 2019.
