The National Institutes of Health (NIH) Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs are congressionally-mandated set-aside programs to encourage research and development that has a strong potential for technology commercialization. These programs were developed to meet the following objectives:
- Stimulate technological innovation;
- Meet federal research and development needs;
- Increase private-sector commercialization of innovations developed through federal R&D funding; and
- Foster and encourage participation in innovation and entrepreneurship by socially and economically disadvantaged persons and women-owned small businesses.
The Small Business Program at the NIBIB is used to achieve the mission of the Institute by supporting innovative technologies through various stages of commercial research and development. The NIBIB welcomes SBIR and STTR applications from small businesses proposing ideas relevant to its scientific program areas. Please contact program staff if you have questions about which Institute(s) would be the best fit for your project.
Program Structure
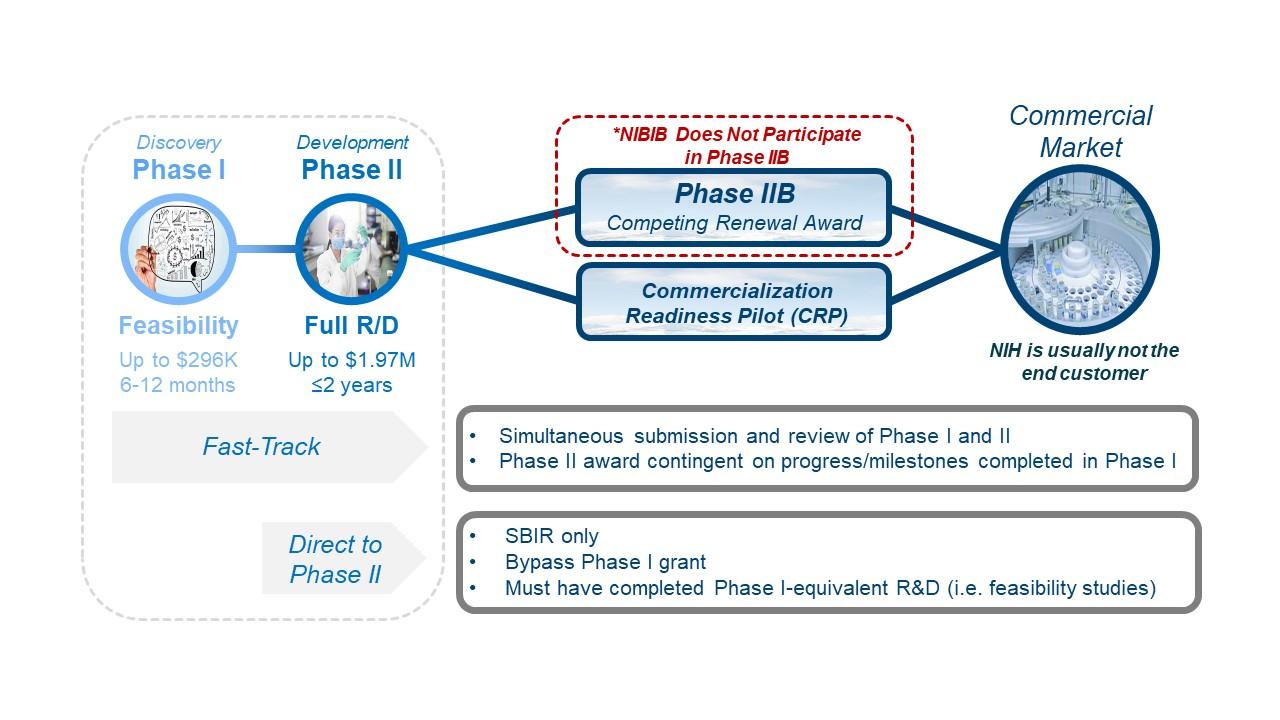
Both the SBIR and STTR programs are divided into three phases as shown in the illustration below.
Webinars

Small Business Program webinar: NIH/NIBIB held an information webinar to announce the Notice of Special Interest to Developers of Technologies for Maternal Health NOT-EB-21-001.
NIBIB held a workshop in 2022 on Technology to Improve Maternal Health.
Information from the 2022 event can be found here.
NIBIB held a Small Business Initiatives webinar:
Funding Opportunities

Current NIH SBIR and STTR funding opportunity announcements
Applicants are strongly encouraged to contact NIBIB staff before submitting an SBIR or STTR application.
Division Staff



Resources
Additional support is available through entrepreneurial training and commercialization assistance resources. Please refer to the programs below for further details and contact program staff with any questions.
Related News
Augmented reality navigation system could improve lumbar puncture accuracy
A team of researchers funded by a NIBIB Small Business program grant developed a new ultrasound navigation system that could provide accurate, real-time, and intuitive needle insertion planning and guidance for lumbar puncture procedures.
Science HighlightsBuilding a better part for your heart
NIBIB-funded engineers are designing aortic heart valve replacements made of polymers rather than animal heart tissues. The goal is to optimize valve performance and enable increased use of a minimally-invasive method for valve replacement over open heart surgery.
Science Highlights

