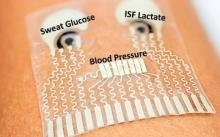
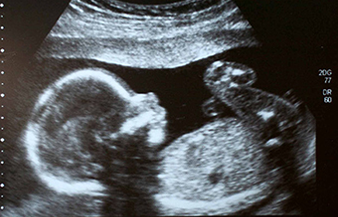
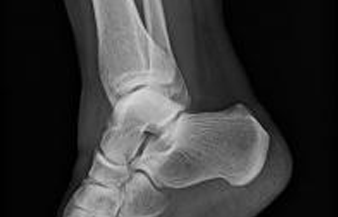
The following fact sheets in PDF format provide an in-depth look at the latest science in medical technology.
![]() Download Adobe Reader | Download Adobe Reader App for Mobile
Download Adobe Reader | Download Adobe Reader App for Mobile
![]() Download all Science Topics Fact Sheets
Download all Science Topics Fact Sheets
Acceda a la versión en español de las hojas informativas sobre temas científicos.