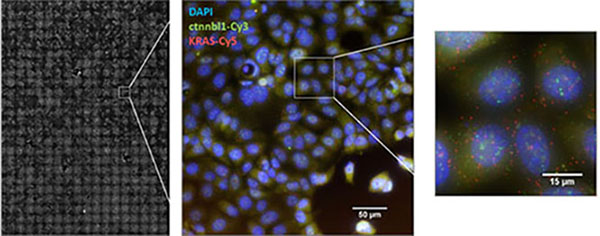
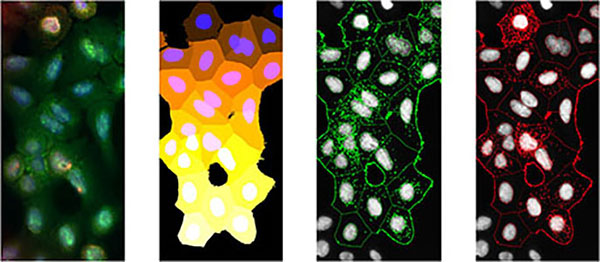
The high throughput single molecule microscope was designed to image large numbers of cell samples in a multi-well format. This microscope can routinely image up to 8 coverslips and 10,000+ cells per acquisition. An included analysis pipeline automatically segments images to identify nuclei, cell bodies, and puncta (RNAs, receptors, etc.) and generates statistics on these puncta (number/cell, co-expression, co-localization, etc.). This microscope is currently located in the Larson Lab and will move to AIM once the facility is operational.
Image Gallery