Three medtech participants share their experiences
The Concept to Clinic: Commercializing Innovation (C3i) program has reached a milestone─its 10-year anniversary.
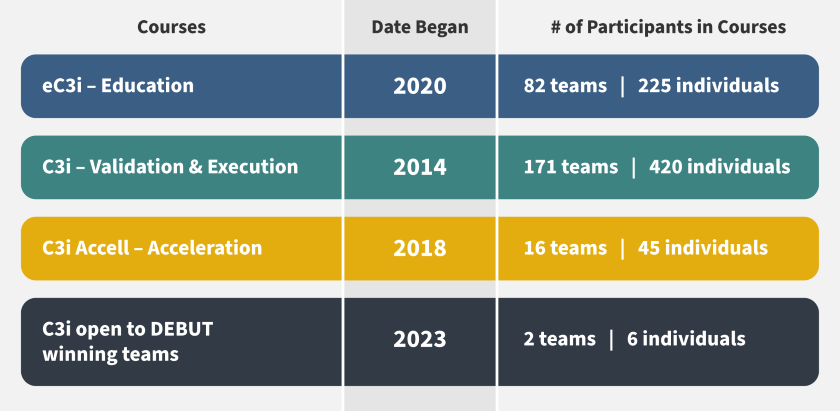
The 24-week intensive C3i course was developed to help medical device innovators translate their technologies from the lab (concept) to the market (clinic). Since launching in 2014, two new courses have been added to the curricula and more than 250 teams and 650 individuals have participated. (see infographic).
“With the C3i curriculum developed by the Wallace H. Coulter Foundation, we saw the clear value of helping biomedical innovators understand and prepare for the rigors of entrepreneurship in the health care arena,” said Todd Merchak, director of NIBIB’s Office of Program Evaluation and Strategic Partnerships, which oversees the program. The C3i curriculum is based on the Coulter Commercialization Process developed at the Coulter Foundation. NIBIB formed a partnership with the foundation in 2014 and Lab2Market (a contractor) has managed the C3i program since then.
“The C3i program represented a novel approach to supporting NIH technology developers on the path to commercialization. We are encouraged by the track record of success to date and hope to continue helping innovators turn their discoveries into useful health care products,” Merchak said.
To mark the 10-year anniversary, we feature three medical technology innovators with academic and/or business backgrounds who participated in the program at the early stages of their biomedical technologies.

One of those participants is Peter Kim, M.D., Ph.D., founder and chief science and medical officer of Activ Surgical, Inc., and a professor of surgery at the State University of New York at Buffalo. He completed the core C3i course in 2018 and his surgical vision device was cleared by the U.S. Food and Drug Administration (FDA) in 2021, enabling him to market and sell it in the U.S.
While Kim was demonstrating the first autonomous robotic surgery of soft tissue with an NIBIB basic research grant in 2016, he realized that a surgical vision tool could have greater impact. He pivoted to bringing that idea to market and received two small business program grants from NIBIB for commercial research and development while he was enrolled in C3i. Kim said that the two NIBIB grants enabled him to conduct proof-of-concept and feasibility studies that helped attract venture capital seed funds.
The core C3i course
“The curriculum and customized mentoring provided by the C3i program are intended to guide innovators as they assess the commercial viability and potential business opportunity for their innovation,” said Kari Ashmont, Ph.D., translational team lead in the NIBIB Office of Program Evaluation and Strategic Partnerships.
Ashmont noted that the number of NIH institutes that participate in funding the core C3i program has grown from two the first year (NIBIB and the National Heart, Lung, and Blood Institute) to nine plus the BRAIN Initiative® this year. Each institute has its criteria for selecting the participating teams’ projects.
The six-month course is divided into two phases: The first three-month phase focuses on characterizing the unmet market need, identifying and interviewing customers and potential partners, and validating the business opportunity.
Kim said, “The C3i program helped us figure out who are customers are and what they wanted. The feedback we received from surgeons led us to refine an aspect of the surgical vision tool. We also learned how to craft a concise, focused narrative about our product.”
The second phase covers intellectual property strategy, regulatory and reimbursement requirements, risk assessment, milestone creation, and preparing to pitch to investors and/or strategic partners.
During the course, Kim engaged regularly with industry business advisors who recommended that he meet with FDA officials before making the product. “Having an in-person pre-submission meeting was helpful because we learned that the FDA didn’t have any objections and that we could proceed down the path to gaining FDA approval,” he said.
He continues to apply the strategies he learned from C3i to take his product to the next level in the marketplace. “I still think about the importance of knowing the value proposition in a competitive market, assessing the risks and determining whether they can be mitigated (derisked),” said Kim.
Academic innovator takes a different approach

Shiva Abbaszadeh, Ph.D., an associate professor in electrical and computer engineering at the University of California at Santa Cruz, decided to take a different approach to commercializing her technology: a dual-layer flat-panel x-ray detector that can score coronary arterial calcium.
Rather than create a startup or a strategic partnership, she plans to remain in academia and license her prototype to a company that is an Original Equipment Manufacturer (OEM), that makes flat-panel x-ray detector products.
Soon after Abbaszadeh received NIBIB funding to develop the technology, she completed eC3i, the online introductory 12-week course aimed at teaching academic innovators methods to assess the commercial value of their biomedical technologies. She then completed C3i last year.
As a researcher, she said that she wouldn’t have thought of interviewing the technology end-users (cardiologists/radiologists) or manufacturers. “That was very helpful because when we talked to the company (manufacturer), they asked us questions about how our prototype would integrate with their existing technology. We realized we hadn’t thought about that aspect and would have to address that,” said Abbaszadeh.
She liked that she started the program before she had built a prototype because she could implement what she learned in real-time. Having a prototype isn’t required to participate in the C3i program.
But there were also drawbacks. “It was frustrating at times when the online exercises and presentations were aimed at participants who had developed their prototypes. But everyone was kind and understanding—they met you where you were.”
She is now completing the year-long C3i Accell course that NIBIB funds, designed for academic innovators who want to accelerate the translation of their biomedical technologies. She received an administrative supplemental grant from NIBIB that allows her to apply lessons learned from the C3i program to complete the proof-of-concept studies that OEM partners require to license her prototype for commercialization.
“One of the most beneficial aspects throughout the C3i program was that I was matched with a business advisor with industry experience in the flat-panel x-ray detector technology that I wanted to license. As an executive at a major U.S. health technology company and start-up founder, he could explain what’s involved in licensing my technology to an OEM and prepare me for a meeting with industry partners,” said Abbaszadeh.
Beyond the classroom: student engineer gains valuable business knowledge
Ali Bisaccia, now a graduate student engineer at Northeastern University, won an NIH Design by Biomedical Undergraduate Teams (DEBUT) Challenge award in 2022 when she was an undergraduate student at Rensselaer Polytechnic Institute in New York. That experience gave her the confidence that she could create devices with market potential, but she also realized that she had little knowledge of the commercialization process.
“The C3i program gives DEBUT Challenge winners the opportunity to continue working on their engineering projects while learning about all aspects of the commercialization process,” said Dave Gutekunst, Ph.D., program director in the NIBIB Division of Interdisciplinary Training that oversees DEBUT.
After winning a DEBUT Challenge award, Bisaccia decided to develop a new company, Xander Medical. The company’s first device is an orthopedic interlock screw that facilitates removing broken or stripped screws used in orthopedic fixation surgeries.
She started C3i last fall with her co-founder and team member Jackson Bisaccia (her brother). In Phase 1, they focused on collecting data from potential users, developing a business model and pitch, and validating a market need for her technology.
“Evaluating the business model and clinical need in Phase 1 was valuable in determining the device’s market potential,” said Bisaccia.
In Phase 2, which began in February, they attended a two-and-a-half day bootcamp in Dallas. That’s where participating companies present their business models, prototypes, and key experiments, receive feedback from industry business advisors, attend roundtable discussions, and speak with other teams about their projects.
Bisaccia said the bootcamp experience was invaluable. “As an early-stage company, the ability to connect with other teams and business advisors in person fostered some of the most valuable feedback we have received thus far. Most of the teams have been developing their technologies for the last few years, all with varying levels of grant, institutional, and venture capital funding. Being able to tap into the combined experience of the teams and business advisors gave us new insights about the best path forward to having a commercially viable product.”
Ultimately, her goal is to license the prototype technology to an orthopedic hardware manufacturer to implement in its existing surgical screw systems. A major advantage is that manufacturers already have the infrastructure and FDA approval pipelines to bring new technologies to market.
In the past decade, the C3i program has expanded to include two new courses (eC3i and Accell) and DEBUT winning teams, which has also boosted participation. Here are the key milestones: