Posted on
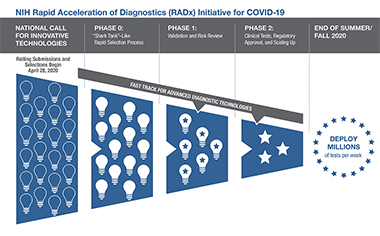 The RADx-tech program offers extensive expertise and support to bring the diagnostic technologies that require additional clinical, regulatory, and commercialization assistance from development to deployment. The RADx-tech program uses a rigorous, rapid-review process that provides independent evaluation of the technology and the potential to scale. The program leverages the long-standing Point-of-Care Technology Research Network (POCTRN), which is run by the National Institute of Biomedical Imaging and Bioengineering. In a process based on an “innovation funnel,” applications move rapidly through multiple review gates that involve increasing selection pressure.
The RADx-tech program offers extensive expertise and support to bring the diagnostic technologies that require additional clinical, regulatory, and commercialization assistance from development to deployment. The RADx-tech program uses a rigorous, rapid-review process that provides independent evaluation of the technology and the potential to scale. The program leverages the long-standing Point-of-Care Technology Research Network (POCTRN), which is run by the National Institute of Biomedical Imaging and Bioengineering. In a process based on an “innovation funnel,” applications move rapidly through multiple review gates that involve increasing selection pressure.
On entering the innovation funnel, each project is evaluated by a team with wide-ranging expertise. Projects that are deemed to be promising enter into to a weeklong intensive review process, which we refer to as a “shark tank” or “deep dive.” In phase 0, multiple expert reviewers provide a detailed assessment of the technology and give critical feedback to the NIH and the project teams. Approximately 15 to 20% of completed RADx-tech applications enter phase 0. For those projects that are successful (25 to 30% of phase 0 projects), detailed, milestone-driven work packages are developed in order for projects to enter phase 1. Technologies are then rigorously tested and validated in the independent POCTRN validation core over a monthlong process to ensure that these new tests meet or exceed their predicted analytic performance. If a project is judged to be successful at that point, rapid scale-up and clinical testing in phase 2 gets under way, with substantial financial assistance provided.
Applications that have been received to date by the RADx-tech program span nearly every stage of technology development, with submissions coming from small and midsize companies, academic laboratories, early-stage start-up companies, and large commercial manufacturers. It is not uncommon for applicants to still be preparing for their EUA submission to the FDA. Promising technologies that have been reviewed by the program include innovations that allow high-throughput, portable, and point-of-care platforms, from CRISPR (clustered regularly interspaced short palindromic repeats) technologies for the detection of viral nucleic acids to lateral flow strips for both nucleic acid and viral antigen testing. Many platforms integrate advanced microfluidic components and state-of the-art readout strategies that use compact optics and electronics. Overall performance, ease of use, and digital reporting are facilitated by smartphone systems that are designed for nonexperts. In addition, alternatives to conventional nasopharyngeal and anterior nasal swab sampling are being explored, and a majority of prototypes involve the use of saliva, oral swabs, and other collection sites.
As of July 13, 2020, a total of 2587 expressions of interest have been received, and more than 600 full applications have been submitted from 41 U.S. states and the District of Columbia. Within 8 weeks after the launch, 27 projects had successfully made it through the shark tank to phase 1, and the entry of the first project into phase 2 is imminent. As technologies and companies go through this process, the RADx program is also seeking to identify testing platforms that may be especially suitable for testing small groups or isolated, underserved populations at point-of-care sites or in rural or remote areas that do not have access to high-throughput robotic testing systems. Technologies that reduce the facility footprint, decrease overall testing complexity, and provide rapid results will be especially helpful, and the program will work to assist in the deployment of these systems in these specialized environments. As part of this process, ease of use, including specimen-collection methods and clear and easy-to-understand instructions to facilitate widespread uptake, will be integrated into the process.
Active collaborations with other government agencies are critical during this process. The NIH is closely coordinating with the Office of the Assistant Secretary for Health, BARDA, and Department of Defense to ensure that each party is aware of the discussions and existing relationships taking place with private-sector companies, to ensure no duplication of funding, to streamline communications, and to leverage the experience, knowledge, and technical capacities of different agencies within the government.
